On August ,10th 2020. Joysbio’s COVID-19 IgG/IgM Rapid Test Kit(Colloidal Gold) has approved by Anvisa in Brazil , by virtue of complete and detailed technical registration information and high quality products,we have passed the Examination of the Brazilian Ministry of Health , and obtained the Brazilian qualified quality system specification certificate,shortly afterwards,COVID-19 IgG/IgM Rapid Test Kit(Colloidal Gold) has passed Testing and review of technical data by Brazilian laboratory, and obtained the registration of ANVISA Brazil, with registration number : 81952720001.

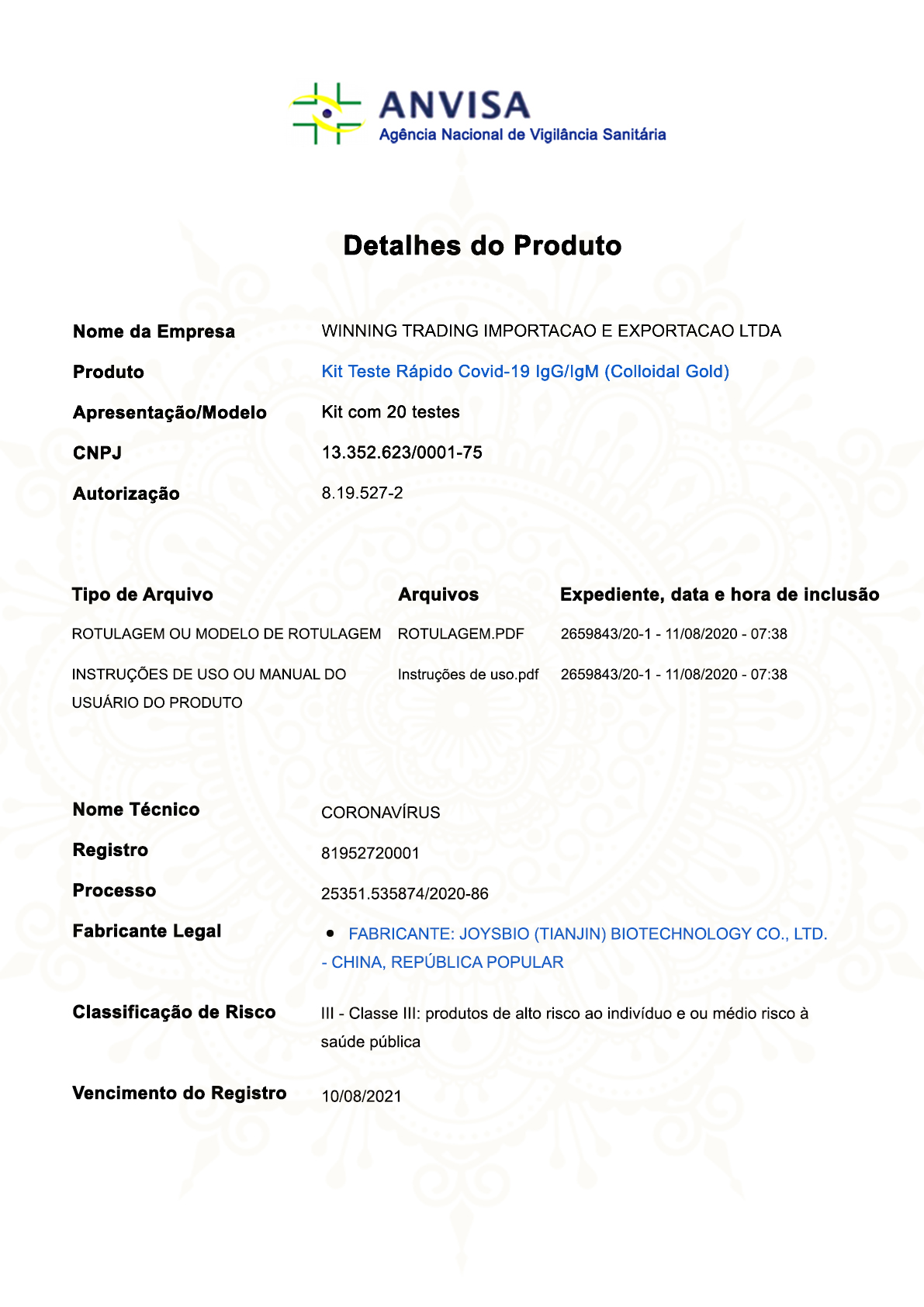
The Brazilian government has strict quality system requirements and product quality requirements for the import of medical devices and in vitro diagnostic reagents.With regard to the epidemic situation this year, the Brazilian government requires more rigorous evaluation on the quality system and product quality of the imported registered manufacturer of relevant medical products.

Under the strict review situation, the successful registration of ANVISA in Brazil is approval of recognition and support for our products in expertise and product quality, and indicates that COVID-19 IgG/IgM Rapid Test Kit(Colloidal Gold) can be sold in the Brazilian market, thereby to make contribute to fight against COVID-19 in brazil!